Describe the Plum Pudding Model of the Atom
Describe the model now used for the structure of an atom. The photon bounces off of the atom.
J J Thomson The History Of The Atom
Circle all correct answers A.
. The model of the atom has changed as. Has electrons outside nucleus in. Plum pudding is an English dessert similar to a blueberry muffin.
Label high-pressure areas with the letter h. In Thomsons plum pudding model of the atom the electrons were. Thomson who invented the electron in the year 1897 suggested the atoms plum pudding model in 1904 which was for including the electron in the atomic model.
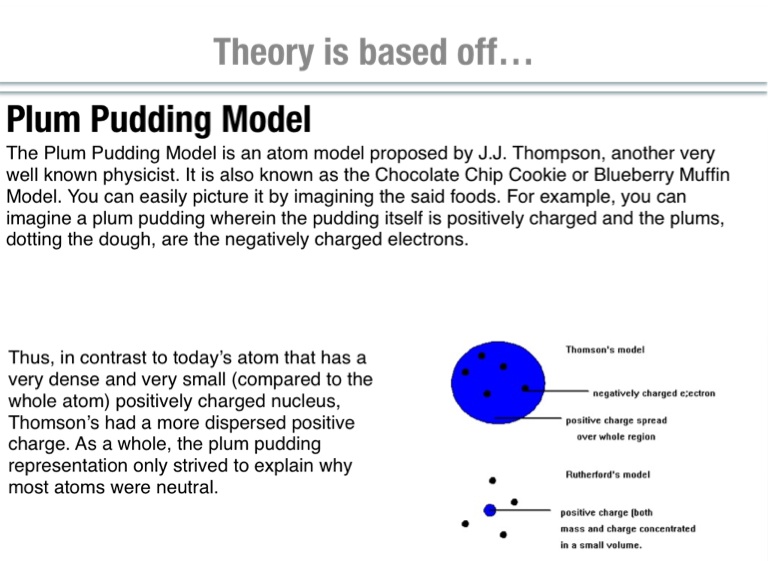
Thomson in 1904 1 soon after the discovery of the electron but before the discovery of the atomic nucleus the model tried to explain two properties of atoms then known. So you have this putting here that is whats the dough of the pudding. No because we have protons neutrons and electrons is the one.
To explain the two. Administrator 2 mins ago Discussion Leave a comment 0 Views. Thompson by the end of the 19th century was a crucial step in the development of atomic physics.
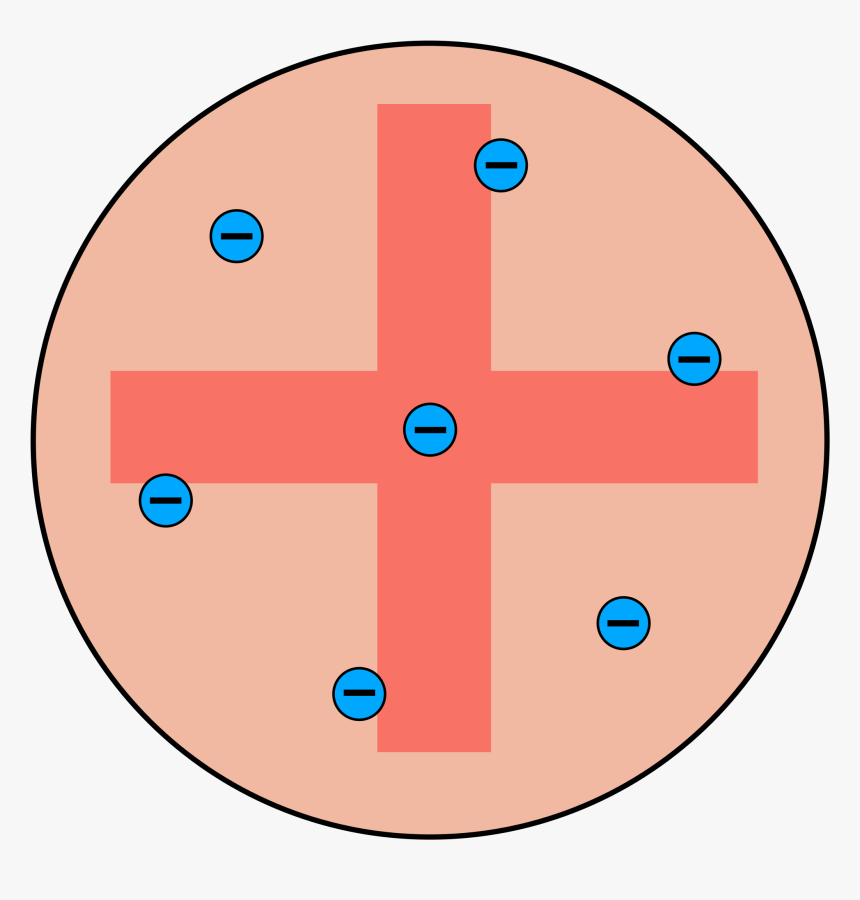
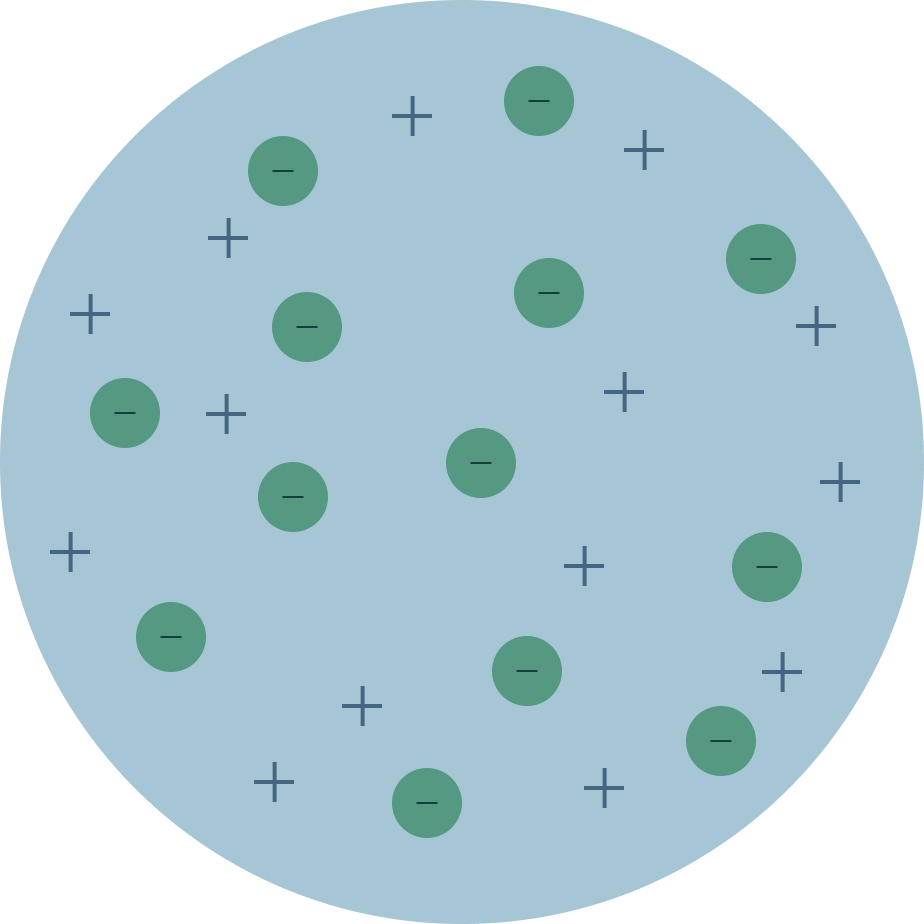
The plum pudding model is defined by electrons surrounded by a volume of positive charge like negatively-charged plums embedded in a positively-charged pudding hence the name. The postulates given by Neils Bohr are. The electron changes position when struck by the photon.
Describe the Plum Pudding Model of the atom. This model is popularly known as plum. The Plum Pudding Model which was devised by JJ.
Home Discussion Describe thomsons plum pudding model of the atom. Thomson developed what became known as the plum pudding model in 1904. Describe thomsons plum pudding model of the atom.
Start studying The Plum Pudding Model. The plum pudding model also known as Thomsons plum pudding model is a historical scientific models of the atom. Nuclear has a positive charged concentrated at the centre plum pudding has a ball of positive charge Nuclear.
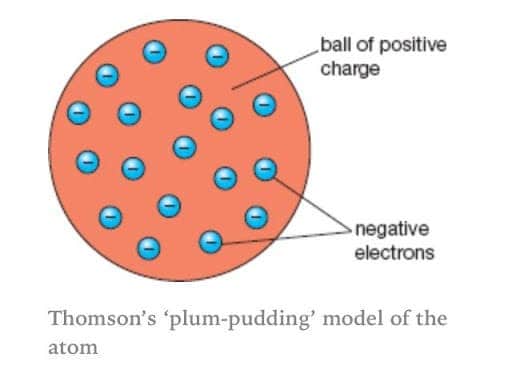
Over which areas would you. Small particles held with in a positively charged sphere. In Thomsons plum pudding model of the atom the electrons were.
Describe the plum pudding model of an atom is this the model of an atom you use today. Advantages of core and shell type transformer. The structure of an atom is explained by JJThomson as the negatively charged electrons were.
Thompson proposed a model of the atom that. The electrons were assumed to be positioned in revolving circles around the atom in this model to be having a cloud of positive charge. Soon after its proposal the model was called a plum pudding model because the positive medium was like a pudding with electrons or plums inside.
Label low-pressure areas with the letter l. The plum pudding model is one of several historical scientific models of the atom. Describe the similarities and differences between the Bohr nuclear model of the atom and the plum pudding model.
Thomson compared plum pudding model with a blue berry muffin where the blue berries are. And this is the positively charged part. That electrons are negatively charged particles and that atoms have no net electric charge.
According to this model the atom is a sphere of positive charge and negatively charged electrons are embedded in it to balance the total positive charge. The plum pudding model also known as Thomsons plum pudding model is a historical scientific models of the atom. What happens when photons interact with the atom in this model.
First proposed by J. So the plum pudding model of the atom is as follows. The plum pudding model.
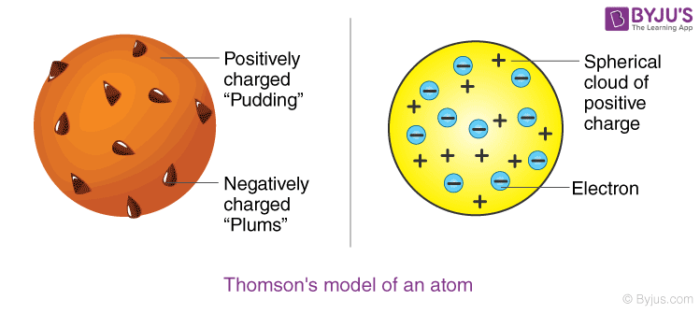
An atom consists of a positively charged sphere with electrons embedded in it. The electrons are like plums in a pudding. Photon is absorbed and a different photon is.
Describe the plum pudding model of the atom. Soon after its proposal the model was called a plum pudding model because the positive medium was like a pudding with electrons or plums inside. How did scientists figure out the structure of atoms without looking at them.
Nuclear model mass is concentrated at the centre nucleus 1 plum pudding model mass is evenly distributed 1 accept the nuclear model has a nucleusthe plum pudding model does not have a nucleus for 1 mark nuclear model positive charge occupies only a. After discovering the electron in 1897 J J Thomson proposed that the atom looked like a plum pudding. Plum pudding is an English dessert similar to a blueberry muffin.
Both contained negatively charged electrons both have positive charges neither has neutrons. The model he proposed was named as plum pudding model of the atom. The plum pudding model of the atom was used by scientists in the early part of the 20th century to explain atomic structure.
Advantages of dancing in the philippines. Thomsons Plum Pudding Model Use words or a drawing to describe this model of the hydrogen atom. Opposite the gold foil is a screen that emits a flash of light when struck by a particle.
His work is based on an experiment called cathode ray experiment. Thomson atomic model is compared to watermelon. An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude.
The key difference between Thomson and Rutherford model of atom is that Thomson model of atom does not contain any details about nucleus whereas Rutherford model of atom explains about the nucleus of an atom. The plum pudding model is defined by electrons surrounded by a volume of positive charge like negatively-charged plums embedded in a positively-charged pudding hence the name. 2 Show answers Another question on Biology.
So the dough has a positive charge and the raisins that air these dots those have a negatively charged Ah those air negatively charged and those represent the electrons. Thomson developed what became known as the plum pudding model in 1904. Learn vocabulary terms and more with flashcards games and other study tools.
Postulates of Thomsons atomic model. Nuclear model Niels Bohrs model. Pudding model plum pudding is an English dessert.
It only had electrons not protons or neutrons.
Caitlyn Johnson Ernest Rutherford Screen 4 On Flowvella Presentation Software For Mac Ipad And Iphone
Thomson Atomic Model Plum Pudding Model Postulates Limitations

Jj Thomson S Plum Pudding Model Youtube

What Is The Plum Pudding Atomic Model Universe Today Plum Pudding Model Atomic Theory Chemistry Activities
The Plum Pudding Model How A Flawed Idea Was Instrumental In Our Understanding Of The Atom

The History Of The Atomic Model Thomson And The Plum Pudding

Plum Pudding Atomic Model By J J Thomson Chemistrygod
Transparent Chemistry Atom Clipart Atomic Model Plum Pudding Hd Png Download Kindpng

What Is The Plum Pudding Atomic Model Universe Today

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Plum Pudding Model Overview Importance Expii

Plum Pudding Model Overview Importance Expii

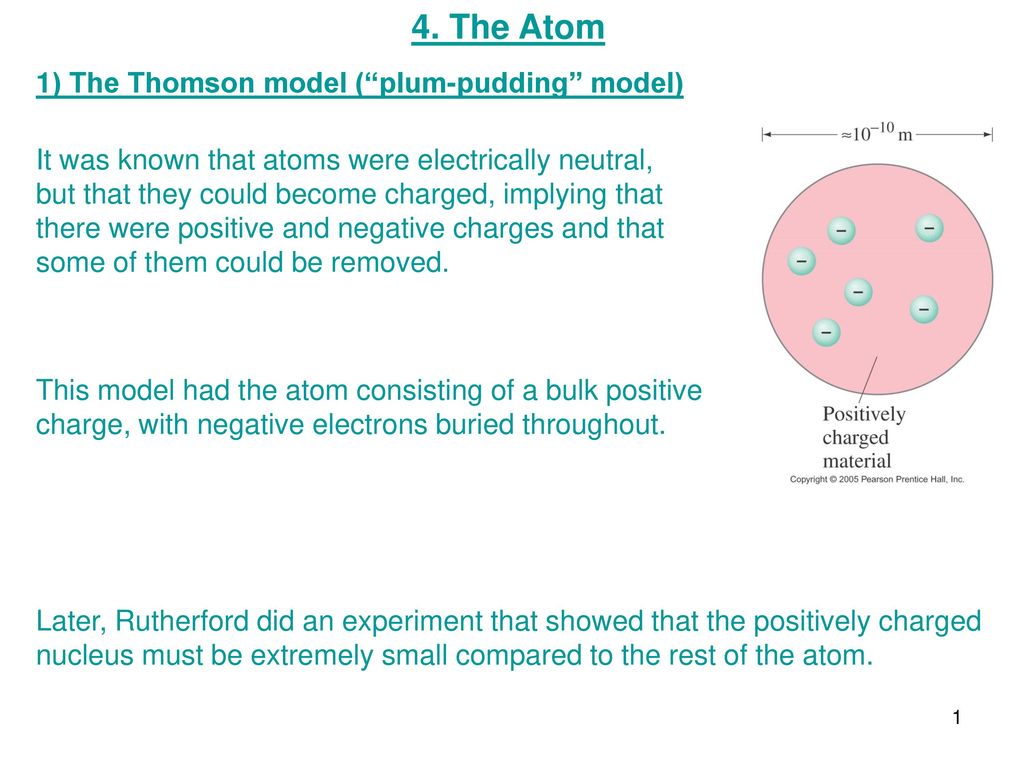
4 The Atom 1 The Thomson Model Plum Pudding Model Ppt Download

The Plum Pudding Model How A Flawed Idea Was Instrumental In Our Understanding Of The Atom
The History Of The Atomic Model Thomson And The Plum Pudding

What Is The Plum Pudding Model
-teachoo.png)
Plum Pudding Model Of Atom Jj Thomson S Model Postulates Limitati

Comments
Post a Comment